Hepatitis C: Del Descubrimiento a la Curación. A Propósito del Premio Nobel de Medicina y Fisiología 2020
Resumen
Se estima que actualmente 71 millones de individuos a nivel mundial son portadores del virus de la hepatitis C (HCV), infección que se asocia en porcentajes variables a la aparición de cirrosis hepática y sus complicaciones al igual que carcinoma hepatocelular (HCC), siendo responsables de aproximadamente 355.000 muertes anuales mundialmente1,2.
El HCV fue descubierto a finales de la década de los 80s, gracias a la admirable labor de los doctores Harvey J. Alter, Michael Houghton y Charles M. Rice, lo que permitió develar la etiología de la mayor parte de los casos de hepatopatía crónica, lo que se tradujo en extensas investigaciones que llevaron en forma progresiva al desarrollo de tratamientos antivirales, que han conducido a la posibilidad cierta de curar definitivamente esta infección.
En este artículo de revisión se describe el camino que llevo desde caracterizar una enfermedad sin agente etiológico (hepatitis NoA-NoB) hasta lograr su curación definitiva, y por supuesto rendir homenaje a los descubridores del HCV, quienes por su labor se hicieron merecedores del Premio Nobel de Medicina y Fisiología del año 2020.
Texto completo:
PDFReferencias
Cacoub J, Saadoun D. N Engl J Med 2021;384:1038-52.
Lauer GM, Walker BD. N Engl J Med 2001:345(1):41-52.
Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med. 1975; 292:767-770.
Alter HJ, Holland PV, Morrow AG, Purcell RH, Feinstone SM, Moritsugu Y. Clinical and serological analysis of transfusion-associated hepatitis. Lancet. 1975; 2:838-841.
Alter HJ, Purcell RH, Holland PV, Popper H. Transmissible agent in non-A, non-B hepatitis. Lancet. 1978; 1:459-463.
Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989; 244:359-362.
Kuo G., Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter CE, Stevens CE, Tegtmeier GE, Bonino F, Colombo M, Lee WS, Kuo C., Berger K, Shuster JR, Overby LR, Bradley DW, Houghton M. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989; 244:362-364.
Kolykhalov AA, Agapov EV, Blight KJ, Mihalik K, Feinstone SM, Rice CM. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997; 277:570-574.
Pawlotsky JM, Feld J, Zeuzem S, Hoofnagle J. Journal of Hepatology 2015 vol. 62 j S87–S99.
Feinstone SM, Kapikian AZ, Purcell RH. Science 1973;182:1026–1028.
Hoofnagle JH, Mullen KD, Jones DB, et al. N Engl J Med 1986;315: 1575–1578.
Press release: The Nobel Prize in Physiology or Medicine 2020 (https://www.nobelprize.org/prizes/medicine/2020/press-release/).
National Institutes of Health Consensus Development Conference Panel: management of hepatitis C. Hepatology 1997;26:5S–10S.
Di Bisceglie AM et al N Engl J Med 1989;321:1506–1510.
Davis GL et al. N Engl J Med 1989;321: 1501–1506.
Poynard et al. Lancet 1998:352;1426-32.
McHutchinson et al. Hepatology 1998:28(4);387A.
Beker B, Leon R et al. GEN 2000:54(3):143-52.
Manns MP et al. Lancet 2001;358:958.
Fried MW et al. N Engl J Med 2002;347:975-982.
Leon R et al. Congreso SVG 2002.
León R et al. Therapies for viral hepatitis. Boston. 2002
EASL HCV Guidelines. Journal of Hepatology 2011: 55:245.64.
Thompson AJ, et al. Gastroenterology. 2010;139:120-129.
Poordad et al. N Engl J Med 2011; 364:1195-1206.
Jacobson I et al. N Engl J Med 2011; 364:2405-2416.
Sherman KE, et al. AASLD 2010. Abstract LB-2.
Leon R et al. Presentación oral en el XXXVI Congreso Venezolano de Gastroenterología 2015.
EASL recommendations on treatment of hepatitis C: Final update of the series. Journal of Hepatology 2020.
Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology 2020 71 (2).
World Health Organization. Global health sector strategy on viral hepatitis 2016-2021 https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06ng.pdf;jsessionid=60A93ADD1A191FF6A0FA823314D24C43?sequence=1(WHO, 2016)..
Ricciardiello L, Ahnen DJ, Lynch PM. Chemoprevention of hereditary colon cancers: time for new strategies. Nat Rev Gastroenterol Hepatol 2016; 13: 352–61.
Holme O, Loberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA 2014; 312: 606–15.
Chiu HM, Chen SL, Yen AM, et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer 2015; 121: 3221–29.
Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;370: 1287–97.
Wiland HOt, Shadrach B, Allende D, et al. Morphologic and molecular characterization of traditional serrated adenomas of the distal colon and rectum. Am J Surg Pathol 2014;38(9):1290-7.
De Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc 2013;77(4):617-23.
Bhurwal A, Bartel MJ, Heckman MG, et al. Endoscopic mucosal resection: learning curve for large nonpolypoid colorectal neoplasia. Gastrointest Endosc 2016; 84: 959–68.
Hayashi N, Tanaka S, Hewett DG, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc 2013; 78: 625–32.
Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2017; 49: 270–97.
Law R, Das A, Gregory D, et al. Endoscopic resection is cost-effective compared with laparoscopic resection in the management of complex colon polyps: an economic analysis. Gastrointest Endosc 2016; 83: 1248–57.
Kontovounisios C, Kinross J, Tan E, et al. Complete mesocolic excision in colorectal cancer: a systematic review. Colorectal Dis. 2015 Jan;17(1):7-16.
Emmanuel A, Haji A. Complete mesocolic excision and extended (D3) lymphadenectomy for colonic cancer: is it worth that extra effort? A review of the literature. Int J Colorectal Dis 2016; 31: 797–804.
Cleary RK, Morris AM, Chang GJ, Halverson AL. Controversies in surgical oncology: does the minimally invasive approach for rectal cancer provide equivalent oncologic outcomes compared with the open approach? Ann Surg Oncol 2018; 25: 3587–95.
Jayne D, Pigazzi A, Marshall H, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA 2017; 318: 1569–80.
Ma B, Gao P, Wang H, et al. What has preoperative radio(chemo) therapy brought to localized rectal cancer patients in terms of perioperative and long-term outcomes over the past decades? A systematic review and meta-analysis based on 41,121 patients. Int J Cancer 2017; 141: 1052–65.
Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29 (suppl 4): 22–40.
Lefevre JH, Mineur L, Kotti S, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol 2016; 34: 3773–80.
van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 2018; 391: 2537–45.
Abdel-Rahman O, Cheung WY. Integrating systemic therapies into the multimodality treatment of resectable colorectal liver metastases. Gastroenterol Res Pract 2018; 2018: 4326082.
Baratti D, Kusamura S, Pietrantonio F, et al. Progress in treatments for colorectal cancer peritoneal metastases during the years 2010–2015. A systematic review. Crit Rev Oncol Hematol 2016; 100: 209–22.
Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27: 3109–16.
Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 2018; 378: 1177–88.
Breugom AJ, Swets M, Bosset J-F, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2015; 16: 200–17.
Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007; 25: 1670–16.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–42.
Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatinbased regimen. J Clin Oncol 2012; 30: 3499–506.
Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versusplacebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after firstline therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 2015; 16: 499–508.
Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017; 28: 1713–29.
Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA 2017; 317: 2392–401.
Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60–00 trial. J Clin Oncol 2010; 28: 466–74.
Adenis A, de la Fouchardiere C, Paule B, et al. Survival, safety, and prognostic factors for outcome with regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 2016; 16: 412.
Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–20.
Bendell JC, Bang Y-J, Chee CE, et al. A phase Ib study of safety and clinical activity of atezolizumab (A) and cobimetinib (C) in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 2018; 36 (suppl 4): 560 (abstr).
Eng C, Kim TW, Bendell J, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2019; 20: 849–61.
Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 2019; 16: 361–75.
Ridolfi T, Berger N, Ludwig K. Low Anterior Resection Syndrome: Current Management and Future Directions. Clin Colon Rectal Surg 2016; 29:239- 45.
Imigo F, Larach J. Síndrome de la resección anterior baja: un alto precio del tratamiento del cáncer de recto. Rev. Cirugia. 2019;71(2).
Lange MM, van de Velde CJ. Urinary and sexual dysfunction after rectal cancer treatment. Nat Rev Urol 2011; 8: 51–57.
Van Blarigan EL, Fuchs CS, Niedzwiecki D, et al. Association of survival with adherence to the American Cancer Society nutrition and physical activity guidelines for cancer survivors after colon cancer diagnosis: the CALGB 89803/alliance trial. JAMA Oncol 2018; 4: 783–90.
Bibbins-Domingo, DC Grossman, SJ Curry, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315 (23): 2564 – 2575.
Rex D, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017 Jul;112(7):1016-1030.
Qaseem A, Crandall C, Mustafa R, et al. Screening for Colorectal Cancer in Asymptomatic Avarage-Risk Adults: A Guidance Statement From the American College of Physicians. Ann Intern Med 2019; 171: 643-54.
DOI: http://dx.doi.org/10.61155/gen.v75i1.546
IMÁGENES GEN
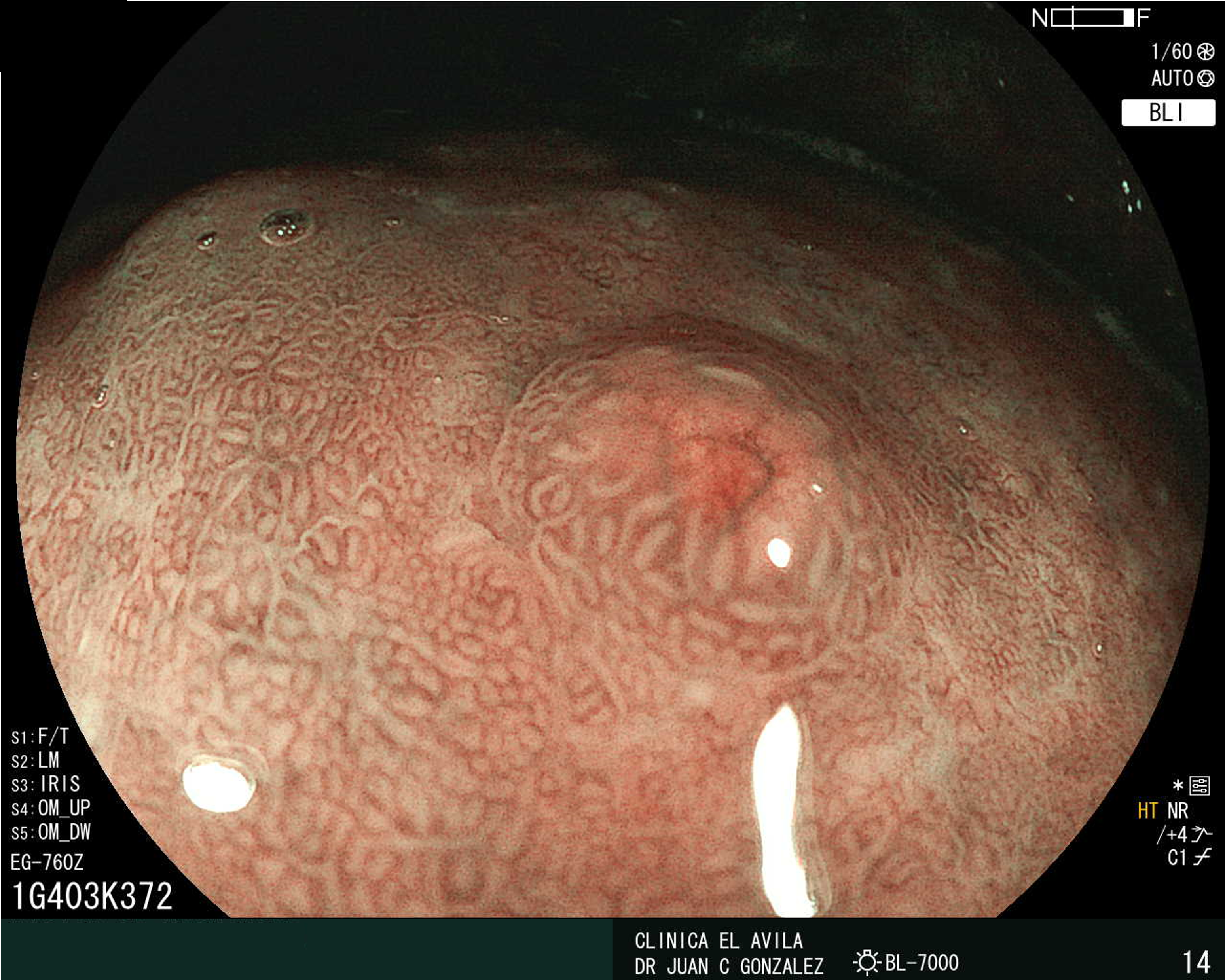
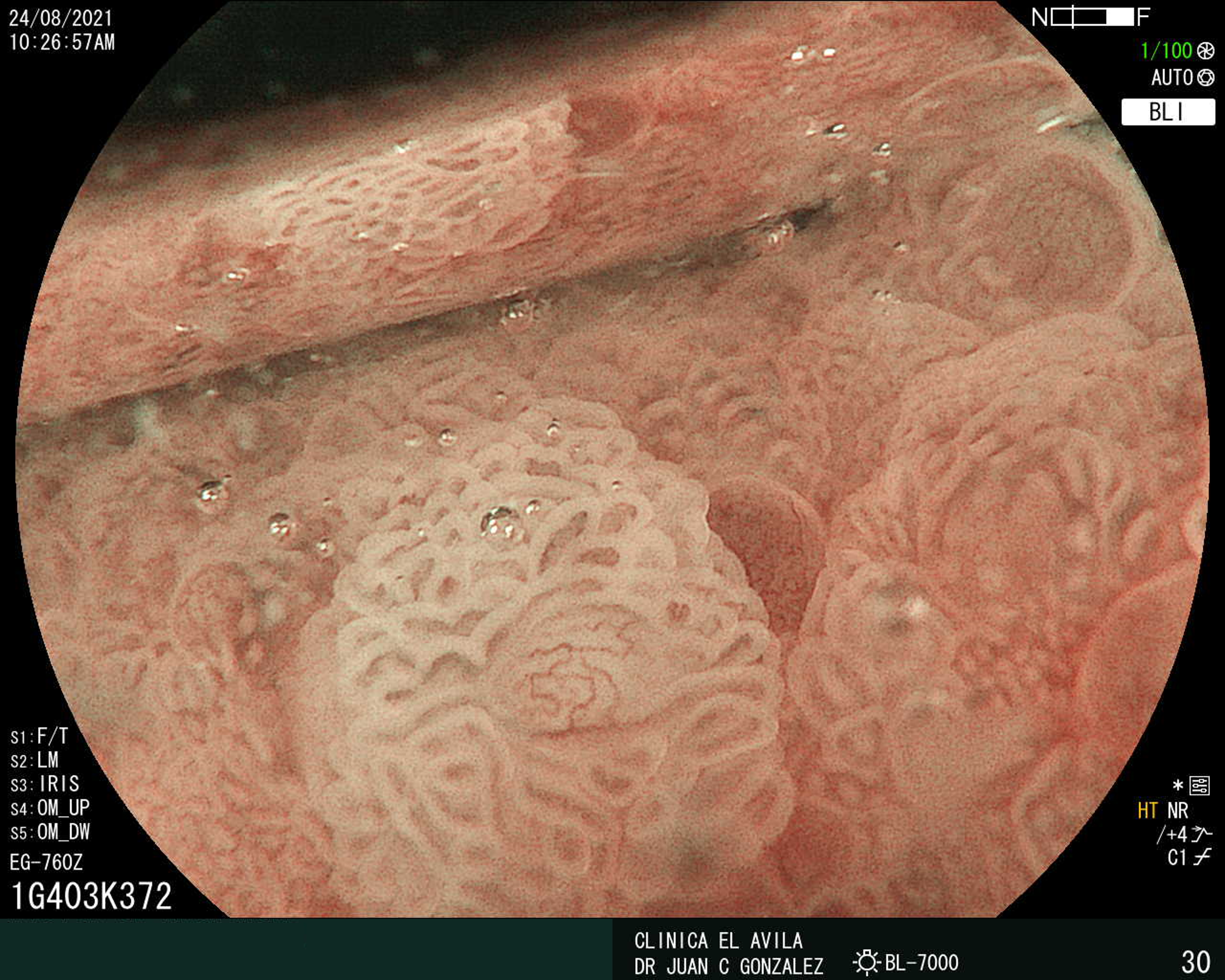
| Figura 1. Tumor Neuroendocrino Gástrico | Figura 2. Hiperplasia de Células Neuroendocrinas en estómago |
 |  |
 |  |  |
ISSN: 0016-3503 e-ISSN: 2477-975X