Terapia convencional en EEI
Resumen
Palabras clave
Texto completo:
PDFReferencias
Pariente B, Cosnes J, Danese S. Development of the Crohn’s Disease Digestive Damage Score, the Lemann Score-Inflamm Bowel Dis 2011;17:1415
Torres J, Billioud V. Sachar DB et al. Ulcerative colitis as a progressive disease: The forgotten evidence. Inflamm Bowel Dis 2012; 18: 1356–1363.
Torres J, Billioud V, Peyrin-Biroulet L et al. Ulcerative colitis as a sole mucosal disease: another misunderstanding? Gut. 2012;61:633.
Sandborn WJ, Hanauer S, Van Assche C et al. Treating beyond symptoms with a view to improving patient outcomes in inflammatory bowel diseases. Journal of Crohn's and Colitis 2014; 8, 927–935
Panaccione R, Colombel JF, Louis E, et al. Evolving Definitions of Remission in Crohn’s Disease. Inflamm Bowel Dis 2013;19:1645–1653
Svartz N. Salazopyrin, a new sulfanilamide preparation. A. Therapeutic results in rheumatic polyarthritis. B. Therapeutic effects in ulcerative colitis. C. Toxic manifestations in treatment with sulfanilamide preparations. Acta Med Scand 1942;110: 580–598
Azad Khan A.K., Piris J., Truelove S.C. An experiment to determine the active therapeutic moiety of sulphasalazine.Lancet 1977;2:892-895.
Caprilli R, J Cesarini M, Angelucci E. Journal of Crohn's and Colitis 2009; 3, 149–156
Hanauer, S, Schwartz, J, Robinson, M. et al, Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. Am J Gastroenterol. 1993;88:1188–1197.
Hanauer SB, Sandborn WJ, Kornbluth A, et al. Delayed-release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: The ASCEND II trial. Am J Gastroenterol. 2005;100:2478–85.
Lichtenstein GR, Ramsey D, Rubin DT. Randomised clinical trial: delayed-release oral mesalazine 4.8 g/day vs. 2.4 g/day in endoscopic mucosal healing—ASCEND I and II combined analysis. Aliment Pharmacol Ther 2011;33:672–8.
Sandborn WJ, Regula J,Feagan B et al. Delayed-Release Oral Mesalamine 4.8 g/day (800-mg Tablet) Is Effective for Patients With Moderately Active Ulcerative Colitis Gastroenterología 2009; 137: 1934-1943
Ford, A. C., Achkar, J. P., Khan, K. J.,et al. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol 2011: 106, 601-16.
Kamm MA, Sandborn WJ, Gassull M, Schreiber S, Jackowski L, Butler T, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology 2007;132:66–75.
Kruis W, Bar-Meir S, Feher J, et al The optimal dose of 5-aminosalicylic acid in active ulcerative colitis: a dose-finding study with newly developed mesalamine. Clin Gastroenterol Hepatol. 2003 Jan;1:36-43.
Safdi AV. Cohen RD. Review article: increasing the dose of oral mesalazine therapy for active ulcerative colitis does not improve remission rates Aliment Pharmacol Ther 2007; 26, 1179–1186.
Gomollón Fernando, García-López Santiago, Sicilia Beatriz. Guía clínica GETECCU del tratamiento de la colitis ulcerosa elaborada con la metodología GRADE Therapeutic guidelines on ulcerative colitis: a GRADE methodology based effort of GETECCU
Dignass AU, Bokemeyer B, Adamek H, Mross M, Vinter-Jensen L, Borner N, et al. Mesalamine once daily is more effective than twice daily in patients with quiescent ulcerative colitis. Clin Gastroenterol Hepatol 2009;7:762–9.
Leifeld L, Pfützer R, Morgenstern J, et al. Mesalazine granules are superior to Eudragit-L-coated mesalazine tablets for induction of remission in distal ulcerative colitis - a pooled analysis. Aliment Pharmacol Ther. 2011;34:1115-22.
Lichtenstein GR, Kamm MA, Boddu P, et al. Effect of once- or twice-daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol 2007;5: 95–102.
Kane, S., Huo, D., Aikens, J et al. 2003. Medication non adherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med, 114, 39-43.
van Bodegraven AA, Boer RO. Sindram JW, et al Distribution of mesalazine enemas in active and quiescent ulcerative colitis. Aliment Pharmacol Ther. 1996;10:327-32.
Safdi M DeMicco M, Sninsky C, et al. A double-blind comparison of oral versus rectal mesalamine versus combination therapy in the treatment of distal ulcerative colitis. Am J Gastroenterol. 1997;92:1867-71.
Marteau P, Probert CS, Lindgren S, Gassul M, et al. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut. 2005;54:960–5
Hanauer. S.Dose-ranging study of mesalamine (Pentasa) enemas in the treatment of acute ulcerative proctosigmoiditis: results of a multicentered placebo-controlled trial: the U.S. PENTASA Enema Study Group. Inflamm Bowel Dis, 1998; 4: 79–83
Hartmann F, Stein J; BudMesa-Study Group Clinical trial: controlled, open, randomized multicentre study comparing the effects of treatment on quality of life, safety and efficacy of budesonide or mesalazine enemas in active left-sided ulcerative colitis. Aliment Pharmacol Ther. 2010;32:3:368-76.
Marshall JK, Irvine EJ. Rectal corticosteroids versus alternative treatments in ulcerative colitis:a meta-analysis. Gut 1997; 40:775.
Nikfar S, Rahimi R, Reizaie A, Abdollahi M. A meta-analysis of the efficacy of sulfasalazine in comparison with 5-aminosalycilates in the induction for improvement and maintenance of remission in patients with ulcerative colitis. Dig Dis Sci. 2009;54, 1157-11-70
Singleton, J. W., Hanauer, S. B., Gitnick, G. L., Peppercorn, M. A., Robinson, M. G., Wruble, L. D. & Krawitt, E. L.. Mesalamine capsules for the treatment of active Crohn's disease: results of a 16-week trial. Pentasa Crohn's Disease Study Group. Gastroenterology1993, 104, 1293-301.
Tremaine, W. J., Schroeder, K. W., Harrison, J. M. & Zinsmeister, A. R.. A randomized, double-blind, placebo-controlled trial of the oral mesalamine (5-ASA) preparation, Asacol, in the treatment of symptomatic Crohn's colitis and ileocolitis. J Clin Gastroenterol, 1994; 19, 278-82.
Hanauer SB, Sandborn W. Practice Parameters Committee of the American College of Gastroenterology Management of Crohn’s disease in adults. Am J Gastroenterol 2001; 96: 635–43
Panaccione R, Rutgeerts P, Sandborn. WV. Review article: treatment algorithms to maximize remission and minimize corticosteroid dependence in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2007, 28, 674–688
Lichtenstein G, Hanauer SB, Sandborn WJ. Management of Crohn ’ s Disease in Adults Am J Gastroenterol 2009; 104:465–483
Hanauer, S. & Stromberg, U.. Oral Pentasa in the treatment of active Crohn's disease: A meta-analysis of double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol, 2004; 2, 379-88.
Messori A, Brignola C, Trallori, et al. Effectiveness of 5-aminosalicylic acid for maintaining remission in patients with Crohn's disease: a meta-analysis. Am J Gastroenterol 1994;89:692–8.
Salomon P, Kornbluth A, Aisenberg J, Janowitz H. - How effective are current drugs for Crohn's disease? A meta-analysis. J Clin Gastroenterol 1992; 14:211-5.
Akobeng A K, Gardener E. Oral 5-aminosalicylic acid for maintenance of medicallyinduced remission in Crohn’s disease. Base de Datos Cochrane Syst Rev 2005; 1: CD003715
Steinhart AH, Hemphill D, Greenberg GR. Sulfasalazine and mesalazine for the maintenance therapy of Crohn's disease: a meta-analysis. Am J Gastroenterol 1994;89:2116–24.
Camma, C., Giunta, M., Rosselli, M. et al. 1997. Mesalamine in the maintenance treatment of Crohn's disease: a meta-analysis adjusted for confounding variables. Gastroenterology, 113, 1465-73.
Steinhart A.H., Forbes A., Mills E.C., et al. Systematic review: the potential influence of mesalazine formulation on maintenance of remission in Crohn's disease. Aliment Pharmacol Ther 25, 1389–1399.
Dignass, A., Lindsay, J. O., Sturm, A., et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis, 6, 991-1030.
Malchow, H., Ewe, K., Brandes, J. W., European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology, 1984; 86, 249-66.
Summers, R. W., Switz, D. M., Sessions, et al. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology, 1979; 77, 847-69.
Taffet S.L. and Das K.M. Sulfasalazine adverse effects and desensitization. Dig. Dis. Sci. 1983; 28:833-842
Das KM, Eastwood MA, Mc Manus JPA and Circus SW. Adverse reactions during salicylazo-sulphapyridine therapy and the relation with drug metabolism and acetylator phenotype. N. Engl J Med 1973; 289:491.
Toth A. Reversible toxic effect of salicylazosulphapyidine on semen quality. Fertil Steril 1979, 31:533-40,
Ransford, R. A. & Langman, M. J.. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. 2002; Gut, 51, 536-9.
Ireland A and Jewell DP. Sulphasalazine and the new salicylates. Eur J Gastroenterol Hepatol 1:43,1989. (35)
Jänerot G. Newer 5 aminosalicylic acid based drugs in chronic inflammatory bowel disease. Drugs 37:73-86,1989.
Hanauer SB, Smith M. Critical drug reappraisal: olsalazine. Drug Ther. Bull. 21:57, 1991. (39)
Loftus, E. V., Jr., Kane, S. V. & Bjorkman, D. 2004. Systematic review: short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther, 19, 179-89.
Van Staa, T. P., Travis, S., Leufkens, H. G. et al 5-aminosalicylic acids and the risk of renal disease: a large British epidemiologic study. Gastroenterology, 2004.126, 1733-9.
Truelove SC, Witts LJ. Cortisone in ulcerative colitis; preliminary report on a therapeutic trial.Br Med J 1954;2:375–8.
Truelove S. Medical management of ulcerative colitis and indications for colectomy. In: Inflammatory Bowel Disease. (Eds. Järnerot G., Lennard-Jones J, Truelove S.) Corona AB and Astra Publishers, Malmo, Sweden,15:361-376, 1992. Rice-Oxley JM, Truelove SC. Ulcerative colitis course andprognosis. Lancet 1950;255:663–6
Rice-Oxley JM, Truelove SC. Ulcerative colitis course and prognosis. Lancet 1950;255:663–6.
Van Assche, G., Dignass, A., Panes et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis, 2010: 4, 7-27.
Munkholm P. Langholz E, Davidsen M et al. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994:35:360–2
Singleton JW, Law DH, Kelley ML Jr et al National Cooperative Crohn's Disease Study: adverse reactions to study drugs. Gastroenterology. 1979;77:870-82
Truelove SC, Witts LJ Cortisone in ulcerative colitis; preliminary report on a therapeutic trial. Br Med J. 1954 14;2:375-8.
Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955;2:1041–8
Lennard-Jones JE, Longmore AJ, Newell AC, Wilson CW, Jones FA. An assessment of prednisone, salazopyrin, and topical hydrocortisone hemisuccinate used as out-patient treatment for ulcerative colitis. Gut 1960;1:217–22.
Truelove SC,Watkinson G, Draper G. Comparison of corticosteroide and sulphasalazine therapy in ulcerative colitis. Br Med J 1962;2:1708–11.
Lennard-Jones JE, Misiewicz JJ, Connell AM, et al.Prednisone as maintenance treatment for ulcerative colitis in remission. Lancet 1965; 1:188–189.
Munkholm P, Michetti P, Probert CS, et al. Best practice in the management of mild-to-moderately active ulcerative colitis and achieving maintenance of remission using mesalazine. Eur J Gastroenterol Hepatol 2010;22:912–6.
Truelove SC, Jewell DP. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet i 1974:1067-70,
Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol 2007;5:103–10.
Janerot G., Rolny P. Sandberg-Gertzén H. Intensive intravenous treatment of ulcerative colitis. Gastroenterology 89:1005-1013,1985.
Sandborn, W. J., Travis, S., Moro, L. et al. Once-daily budesonide MMX(R) extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology, 2012, 143, 1218-26 e1-2.
Marshall JK, Irvine EJ. Rectal corticosteroids versus alternative treatments in ulcerative colitis: a meta-analysis. Gut 1997;40:775–81.6.
Danielsson A, Hellers G, Lyrenas E et al. A controlled randomized trial of budesonide versus prednisolone retention enemas in active distal ulcerative colitis. Scand J Gastroenterol. 1987;22:987-92. .
Hanauer SB, Robinson M, et al. Budesonide enemas for the treatment of active distal ulcerative colitis and proctitis:
Sandborn WJ, Bosworth B, Zakko S et al. Budesonide foam induces remission in patients with mild to moderate ulcerative proctitis and ulcerative proctosigmoiditis- Gastroenterology. 2015, 148:740-750.
Faubion WA Loftus EV Jr, Harmsen WS et al. The natural history of corticosteroid therapy for IBD: a population-based study. Gastroenterology 2001; 121: 255-260.
Steinhart, A. H., Ewe, K., Griffiths, A. M.et al. Corticosteroids for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev, 2003 CD000301.
Otley, A. & Steinhart, A. H. 2005. Budesonide for induction of remission in Crohn's disease. Cochrane Database Syst Rev, CD000296.
Rutgeerts, P., Löfberg, R., Malchow, H., Lamers, C., Olaison, G., Jewell, D. et al. (1994) A comparison of budesonide with prednisolone for active Crohn’s disease. N Eng J Med 331: 842–845.
Prantera C. Glucocorticosteroids in the treatment of inflammatory bowel disease and approaches to minimizing systemic activity. Ther Adv Gastroenterol (2013) 6(2) 137–156
Benchimol, E., Seow, C., Otley, A. and Steinhart, A. (2009) Budesonide for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev (1): CD002913.
Lindmark B. PhD, MD. Short and term steroid side effects: the importance for patients with inflammatory bowel disease. In: Reviewing steroids in the treatment of Inflammatory Bowel Disease, Research and Clinical Forums. Ed. Wells Medical England, vol 15, n 5, 35-41,1993.(86)
Toruner, M., Loftus, E. V., Jr., Harmsen, W. S. et. Al J Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology, 2008. 134, 929-36.
Lichtenstein, G. R., Feagan, B. G., Cohen, R. D et al.. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol, 2006; 4, 621-30.
Nielsen OH, Vainer B, Rask-Madsen J.Review article: the treatment of inflammatory bowel disease with 6-mercaptopurine or azathioprine. Aliment Pharmacol Ther. 2001;15:1699-708.
Present DH, Meltzer SJ, Krumholz MP and Wolke A. 6-MP in management of inflammatory bowel disease. Short and long term toxicity. Ann Intern Med 111:641, 1989.
Sandborn WJ. A review of immune modifier therapy for inflammatory bowel disease: azathioprine,6-mercaptopurine, cyclosporine, and methotrexate. Am J Gastroenterol. 1996; 91:423-33.
Dubinsky MC. Optimizing immunomodulator therapy for inflammatory bowel disease. Curr Gastroenterol Rep. 2003;5:506-11. Review.
Cuffari C, Hunt S, Bayless T. Utilisation of erythrocyte 6-thioguanine metabolite levels to optimize azathioprine therapy in patients with inflammatory bowel disease. Gut 2001;48:642-6.
Sandborn WJ. Pharmacogenomics and IBD: TPMT and thiopurines. Inflamm Bowel Dis. 2004 Feb;10 Suppl 1:S35-7.
Frei P, Biedermann L, Nielsen OH et al. Use of thiopurines in inflammatory bowel disease. World J Gastroenterol 2013 19: 1040-1048.
Colombel JF, Ferrari N, Debuysere H, Marteau P, Gendre JP, Bonaz B, Soule JC, Modigliani R, Touze Y, Catala P, Libersa C, Broly F. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn's disease and severe myelosuppression during azathioprine therapy. Gastroenterology 2000;118:1025-30.
Korelitz BH. Antimetabolites in Inflammatory Bowel Disease. The Mount Sinai Journal of Medicine 57:297 304,1990.
Toruner M, Loftus EV Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008;134:929–36
Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn's disease: benefits outweigh the risk of lymphoma. Gastroenterology 2000;118:1018-24.
Fraser AG, Jewell DP. Long-term risk of malignancy after treatment of inflammatory bowel disease with azathioprine
Louis E, Irving P, Beaugerie L et al. Use of Azathioprine in IBD: Modern Aspects of an Old Drug. Gut. 2014;63:1695-1699.
Korelitz BI, Mirsky FJ, Fleisher MR, et al. Malignant neoplasms subsequent to treatment of inflammatory bowel disease with 6-mercaptopurine. Am J Gastroenterol 1999; 94:3248 – 3253.
Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut 2005;54:1121–1125
Beaugerie L, Brousse , Bouvier AM et al; CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009; 374: 1617-1625
Siegel Ca, Marden SM,Persing SM et al. Risk of Lymphoma Associated With Combination Anti–Tumor Necrosis Factor and Immunomodulator Therapy for the Treatment of Crohn’s Disease: A Meta-Analysis. Clinical Gastroenterology and Hepatology 2009;7:874–881
Navarro JT, Ribera JM, Mate JL, et al. Hepatosplenic T-gamma delta lymphoma in a patient with Crohn's disease treated with azathioprine. Leuk Lymphoma. 2003;44:531–533
Rosh JR, Gross T, Mamula P. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn's disease: a cautionary tale? Inflamm Bowel Dis. 2007;13:1024-30.
Peyrin-Biroulet, L. et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology (2011. 141, 1621–1628
Bo Shen, Feza H. Remzi, et al. A Proposed Classification of Ileal Pouch Disorders and Associated Complications After Restorative Proctocolectomy Clin Gastroenterol Hepatol 2008;6:145–158
Gisbert JP, Linares PM, McNicholl AG, et al. Meta-analysis: efficacy of azathioprine and mercaptopurine in ulcerative colitis Aliment Pharmacol Ther 2009; 30:126-137.
Timmer A, McDonald JW, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerat0ive colitis. Cochrane Database Syst Rev 2007: CD000478.
Ohno K, Masunaga Y, Ogawa R, et al. [A systematic review of the clinical effectiveness of azathioprine in patients with ulcerative colitis]. Yakugaku Zasshi 2004; 124: 555-560.
Ardizzone S, Maconi G, Russo A et al. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut 2006; 55: 47-53
Sambuelli A, Gil AH, Negreira S et al- Steroid-Dependent UC. Colectomy Risk at 5 Years Follow-up With Thiopurine Therapy. Gastroenterology. 2010, Volumen 138, 5, Pag S-692
Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut 2002; 50: 485-489.
Colombel, J. F., Sandborn, W. J., Reinisch, W., et al Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med, 2010, 362, 1383-95.Infliximab, Azathioprine, or Combination Therapy for Crohn's Disease. N Engl J Med 2010; 362:1383-139
Panaccione R, Ghosh S, Middleton S et al Combination Therapy With Infliximab and Azathioprine Is Superior to Monotherapy With Either Agent in Ulcerative Colitis. Gastroenterology 2014;146:392–400
Sandborn, W., Sutherland, L., Pearson, D. Et Al. Azathioprine or 6-mercaptopurine for inducing remission of Crohn's disease. Cochrane Database Syst Rev, 2000. CD000545.
Pearson, D. C., May, G. R., Fick, G. H. Et Al. 1995. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med, 123, 132-42.
Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980 May 1;302(18):981–987.
Prefontaine E, Sutherland LR, Macdonald JK, et al.Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2009 Jan 21;CD000067.
Peyrin-Biroulet L, Deltenre P, Ardizzone S,D'Haens G, Hanauer SB, Herfarth H. et al. Azathioprine and 6-mercaptopurine for the prevention of postoperative recurrence in Crohn's disease: a meta-analysis. Am J Gastroenterol 2009; 104: 2089-96
De Cruz, P., Kamm, M. A., Prideaux, et al. Mucosal healing in Crohn's disease: a systematic review. Inflamm Bowel Dis, 19, 429-44.
Mantzaris GJ, Christidou A, Sfakianakis M et al. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn's disease. Inflamm Bowel Dis. 2009: 15:375-82.
D'Haens G, Geboes K, Ponette E. Healing of Severe Recurrent Ileitis With Azathioprine Therapy in Patients With Crohn’s Disease Gastroenterology 1997;112:1475–1481
Dignass A., Van Assche G., Lindsay JO et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: Current Management. Journal of Crohn's and Colitis (2010) 4, 28–62
Swaminath A, Taunk R, Lawlor G et al.. Use of methotrexate in inflammatory bowel disease in 2014: A User’s Guide. World J Gastrointest Pharmacol Ther 2014; 5: 113-121
Fraser, A. G. 2003. Methotrexate: first-line or second-line immunomodulator? Eur J Gastroenterol Hepatol, 15, 225-31.
Feagan, B. G., Rochon, J., Fedorak, R. N., et al. Methotrexate for the treatment of Crohn's disease. The North American Crohn's Study Group Investigators. N Engl J Med, 1995.332, 292-7.
Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis.Br J Rheumatol. 1997;36:86-90.
Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med 2000; 342: 1627-1632
Mahadevan U, Marion JF, Present DHAbstract Fistula response to methotrexate in Crohn's disease: a case series. Aliment Pharmacol Ther. 2003; 15;18:1003-8.
McDonald JW, Tsoulis DJ, Macdonald JK et al. for induction of remission in refractory Crohn’s disease.Cochrane Database Syst Rev 2012; 12: CD003459
Lémann M, Zenjari T, Bouhnik Y et al- Methotrexate in Crohn’s disease: long-term efficacy and toxicity. Am J Gastroenterol 2000; 95: 1730-1734
Feagan B, MacDonald JWD, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn's disease. Gastroenterology. 2014;146:681-688.
Carbonnel1, J.F. Colombel2, J. Filippi3, Methotrexate for corticosteroid-dependent ulcerative colitis: results of a placebo randomized controlled trial. . www.ecco-ibd.eu/index.php/publications/congress-abstract-s/abstracts-2015- OP023, ECCO Congress Barcelona
Lichtiger S. Cyclosporin therapy in inflammatory bowel disease: open-label experience. Mt Sinai J Med 57:315-319,1990.
Lichtiger, S., Present, D. H., Kornbluth, A et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. 994. N Engl J Med, 330, 1841-5.
Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999;94:1587–92.
Moskovitz DN, Van AG, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760–5Carbonnel F, Boruchowicz A, Duclos B, et al.
Lemann M, et al. Intravenous cyclosporine in attacks of ulcerative colitis: short-term and long-term responses. Dig Dis Sci 1996;41:2471–6.
Actis GC, Fadda M, David E, Sapino A. Colectomy rate in steroid refractory colitis initially responsive to cyclosporin: a long-term retrospective cohort study. BMC Gastroenterol 2007;7:13.
D'Haens G, Lemmens L, Geboes K, Vandeputte L, Van AF, Mortelmans L, et al. Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology 2001;120:1323–9.
Van Assche G, D'Haens G, Noman M, Vermeire S, Hiele M, Asnong K, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology 2003;125:1025–31.
Domenech E, Garcia-Planella E, Bernal I, et al. Azathioprine without oral ciclosporin in the long-term maintenance of remission induced by intravenous ciclosporin in severe, steroid-refractory ulcerative colitis. Aliment Pharmacol Ther 2002;16:2061–5.
Sternthal, M. B., Murphy, S. J., George, et al 2008. Adverse events associated with the use of cyclosporine in patients with inflammatory bowel disease. Am J Gastroenterol, 103, 937-43.
Laharie D, Bourreille A, Branche A, et al. Cyclosporin versus infliximab in severe acute ulcerative colitis refractory to intravenous steroids: a randomized study (CYSIF). J Crohns Colitis 2011;5.
Brynskov J, Freund L, Rasmussen SN, et al. A placebo-controlled,double-blind, randomized trial of cyclosporine therapy in active chronic Crohn's disease. N Engl J Med 1989;321:845–50.
Feagan BG, McDonald JW, Rochon J, et al. Low-dose cyclosporine for the treatment of Crohn's disease. TheCanadian Crohn's Relapse Prevention Trial Investigators. N Engl J Med 1994;330:1846–51.
Jewell DP, Lennard-Jones RE. Oral cyclosporine for chronic active Crohn's disease: a multi-centre controlled trial. Eur J Gastroenterol Hepatol 1994;6:499–506.
Stange EF, Modigliani R, Pena AS, et al. European trial of cyclosporine in chronic active Crohn's disease: a 12-month study. The European Study Group. Gastroenterology 1995;109:774–82.
Systematic review: the role of tacrolimus in the management of Crohn’s disease K. McSharry, A. M. Dalzell, K. Leiper et al. Aliment Pharmacol Ther 2011; 34: 1282–1294
Colombel, J. F., sandborn, W. J., Rutgeerts, P., et al.. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 2007, 132, 52-65.
Sutherland LR, Singloeton J, Sessions J ate al. Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut. 1991;32:1071-5.
Ursing B, Alm T, Barany F, et al. A comparative study of metronidazole and sulfasalazine for active Crohn's disease: the cooperative Crohn's disease study in Sweden. Gastroenterology 1982;83:550–62. 51.
Sandborn W. J ., Feagan B. G. , Lichtenstein GR Medical management of mild to moderate Crohn’s disease: evidence-based treatment algorithms for induction and maintenance of remission_
Colombel JF, Lemann M, Cassagnou M, et al. Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives (GETAID). A controlled trial comparing ciprofloxacin with mesalazine for the treatment of active Crohn's disease. Am J Gastroenterol 1999;94:674–8.
Prantera C, Zannoni F, Scribano ML, et al. An antibiotic regimen for the treatment of active Crohn's disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. Am J Gastroenterol 1996;91:328–32.
Pieter Dewint1, Bettina E Hansen et al. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn's disease: a randomised, double-blind, placebo controlled trial (ADAFI Gut 2014;63:292-299
Solomon M, Mcleod R, O’Conner B, Steinhart AH, Greenberg G, Cohen Z. Combination ciprofloxacin and metronidazole in severe perinanal Crohn’s disease. Can J Gastroenterol. 1993;7:571–3.
Bernstein LH, Frank MS, Brandt LJ et al.. Healing of perineal Crohn's disease with metronidazole. Gastroenterology. 1980;79:357-65.
Brandt LJ, Bernstein LH, Boley SJ, et al. Metronidazole therapy for perineal Crohn's disease: a follow-up study. Gastroenterology.1982; 83: 383–387.
Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology. 1995;108:1617–21.
Rutgeerts P , Van Assche G , Vermeire S et al. Ornidazole for prophylaxis of postoperative Crohn’s disease recurrence: a randomized, double-blind, placebo-controlled trial . Gastroenterology 2005 ; 128 : 856 – 61
De Cruz P, Kamm MA, Hamilton AL, et al. Crohn's disease management after intestinal resection: a randomised trial. The Lancet , 2015; 385:1406 - 1417
Torres J, Caprioli F, Katsanos KH, et al. Predicting Outcomes to Optimize Disease Management in Inflammatory Bowel Diseases. J Crohns Colitis. 2016;10(12):1385-1394.
Beaugerie L, Seksik P, Nion-Larmurier I et al. Predictors of Crohn's disease. Gastroenterology. 2006;130:650-6.
Bouguen G, Levesque BG, Feagan BG et al. Treat to Target: A Proposed New Paradigm for the Management of Crohn's Disease.Clin Gastroenterol Hepatol. 2013 Sep 10. pii: S1542-356501301
DOI: http://dx.doi.org/10.61155/gen.v73i2.488
IMÁGENES GEN
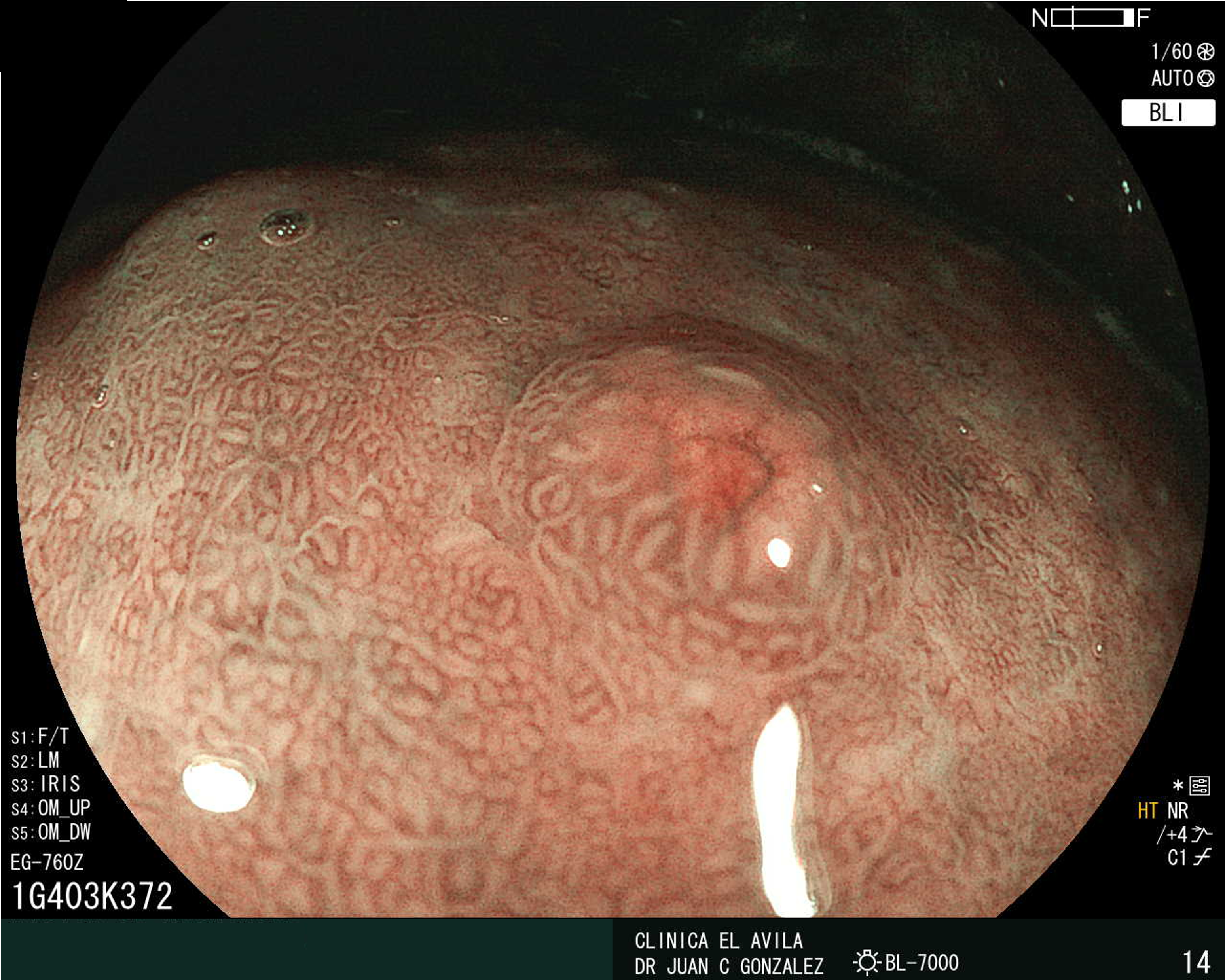
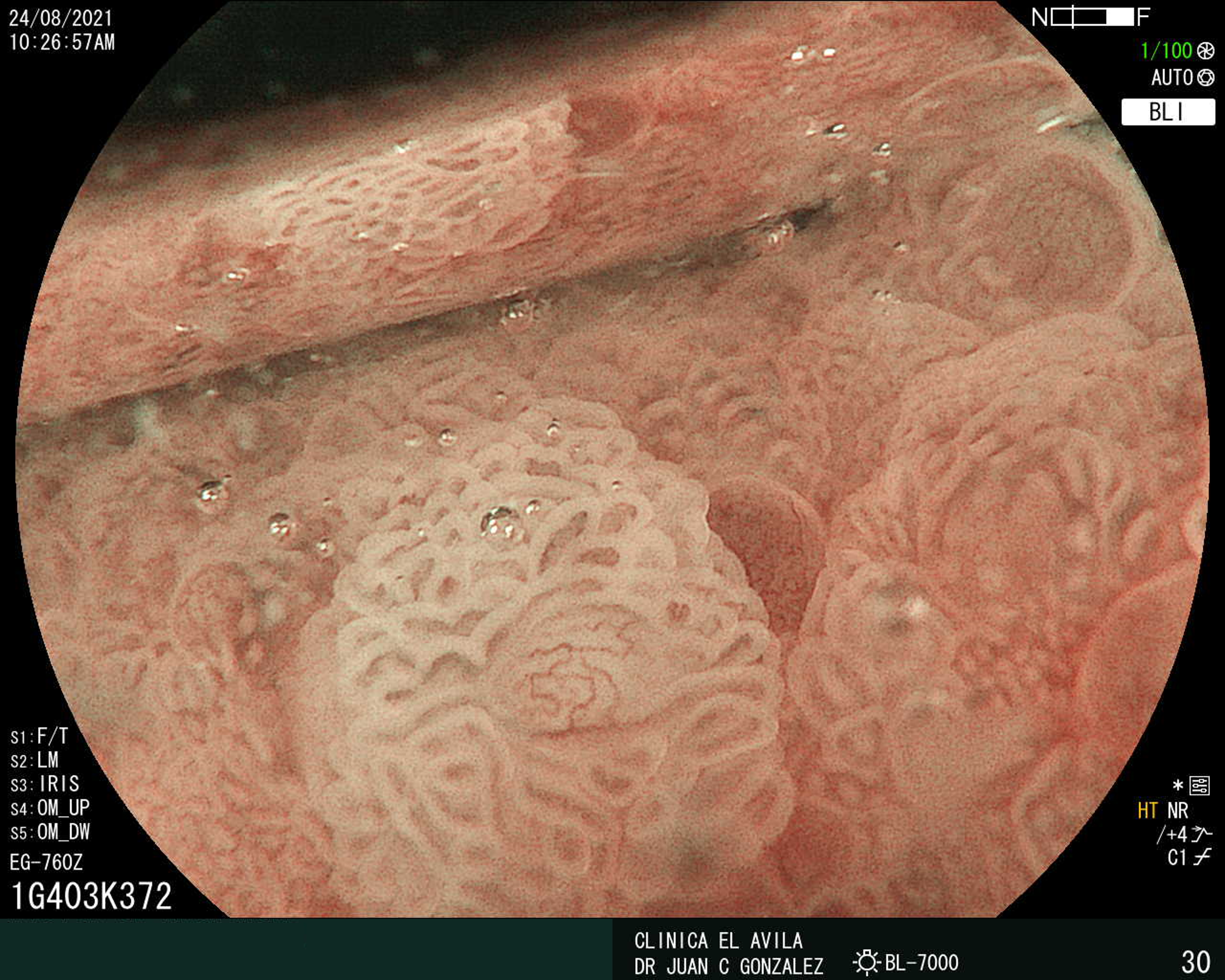
| Figura 1. Tumor Neuroendocrino Gástrico | Figura 2. Hiperplasia de Células Neuroendocrinas en estómago |
 |  |
 |  |  |
ISSN: 0016-3503 e-ISSN: 2477-975X