GUIA NACIONAL DE TRATAMIENTO PARA PACIENTES CON HEPATITIS C EN VENEZUELA 2016
Resumen
Dentro del marco científico de la Sociedad Venezolana de Gastroenterología (SVG) (Sección de Hepatología y Sección de Educación Médica), y en nuestro constante interés por mantener la actualización en temas de renovado tratamiento, decidimos emprender la tarea de elaborar la Guía nacional de tratamiento para pacientes con hepatitis C (GNTHC) para que los Médicos y expertos de diferentes especialidades involucrados en el diagnóstico, tratamiento y seguimiento de pacientes infectados con el virus de Hepatitis C, incluyendo a Médicos Generales, Internistas, Pediatras, Gastroenterólogos, Hepatólogos, Hematólogos, Oncólogos, Infectólogos, Psiquiatras y profesionales de los Servicios de Bancos de Sangre y Trasplantes de Órganos, Personal de Enfermería, tuviesen una herramienta confiable para el adecuado manejo de sus pacientes, dado que el abordaje integral de esta patología es clave para el éxito de la terapia
En los últimos años ha resurgido el interés por esta patología, debido al auge en la investigación con nuevos medicamentos que permiten acortar las terapias anteriores y proporcionan un porcentaje mayor de respuesta al tratamiento.
Texto completo:
PDFReferencias
Wilczynski NL, McKibbon KA, Haynes RB. Enhancing retrieval of best evidence for health care from bibliographic databases: calibration of the hand search of the literature. Medinfo 2001;10:390-393.
Montori VM, Wilczynski NL, Morgan D et al. Initial search strategies for retrieving systematic reviews from Medline: analytical survey. BMJ 2005; 330 (7482): 68.
Schünemann HJ, Oxman AD, Brozek J et al. GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008; 336(7653): 1106-1110.
Atkins D, Best D, Briss PA et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004; 328 (7454): 1490-1494.
Atkins D, Eccles M, Flottorp S et al. GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations. Critical appraisal of existing approaches. GRADE Working Group. BMC Health Serv Res 2004; 4(1): 38.
Guyatt GH, Oxman AD, Kunz R et al; GRADE Working Group. Rating quality of evidence and strength of recommendations: Incorporating considerations of resources use into grading recommendations. BMJ 2008; 336: 1170-1173.
Jaeschke R, Guyatt GH, Dellinger P et al. GRADE working group. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 2008; 337: 744-749.
Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107-115
Messina JP, Humphreys I, Flaxman A, et al. Hepatology 2015;61:77-87
Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090-1101
Xia X, Luo J, Bai J, Yu R. Epidemiology of hepatitis C virus infection among injection drug users in China: systematic review and meta-analysis. Public Health. 2008;122:990-1003
Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48:148-162.
Uddin G, Shoeb D, Solaiman S, et al. Prevalence of chronic viral hepatitis in people of south Asian ethnicity living in England: the prevalence cannot necessarily be predicted from the prevalence in the country of origin. J Viral Hepat. 2010;17:327-335
Ponziani FR, Gasbarrini A, Pompili M, Burra P, Fagiuoli S. Management of hepatitis C virus infection recurrence after liver transplantation: an overview. Transplant Proc 2011;43:291-295.
Lorna M. Duve. A general approach to the management of chronic HC. Gastroenterology Clinics 2.004; 33(4):463-477
Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513-521
CDC. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR 2012;61(No. RR-4)
Machado I. Epidemiology of Hepatitis C Virus in South America. American College of Gastroenterology 2.004; 55-56
Muller G, Zabaleta M, Caldera L, Bianco N, Machado I. Hepatitis C en Venezuela. Comunicación preliminar. GEN. 1990;4:(4):336-42
Albornoz A, Esaa R, Rodríguez L, Mujica C. Anti-HVC en pacientes sin factores de riesgo para hepatitis C. Estudio prospectivo en 200 pacientes. GEN.1993; 47:(3) 136-8
Márquez M, Galíndez E, Camacho G, et al. Epidemiología de la hepatitis viral en Venezuela: Resultados preliminares de la etapa I. Prevalencia en el área metropolitana. GEN 1993;47:(4)215-20
Delgado F, Wever W, Fattah K, Mora O, Bongioanni H, Rodríguez De León L. Incidencia del virus de la hepatitis C en una población controlada sin factores de riesgo. GEN 2006; 60(1): 37-41
Bello G. F, González H, Hinestrosa H, Rodríguez De León L, Uribe M y cols. Incidencia de Hepatitis B y C en Núcleos de Población Rural Estado Miranda. GEN 2011; 65(3):283
González I, Romer H, Torres P, et al. Hepatitis viral en niños. Experiencia en un servicio de gastroenterología pediátrica. Revisión de 14 años. GEN. 1998; 52:(3) 214
Lizarzabal M, Romero G, Rangel R, Fernández J , et al. Características seroepidemiológicas y factores de riesgo de infección por virus de hepatitis B y C en personal de salud y población general. H.U.M. Año 2.000. GEN.2002;56(2) 84-94
Páez R, León R, Zuramay C, Aparcero M, Hinestrosa H, Quiroz E y cols. Causa de enfermedad hepática aguda y crónica en 3 consultas de referencia en Hepatología en la ciudad de Caracas. GEN 2003; 57, Nº ESPECIAL:23-26
Cuadra-Sánchez C, Moronta-Piñango R, Córdova-Villanueva E, Mindiola-Morles R y cols. Seroprevalencia del Virus de Hepatitis C (VHC) en pacientes del Laboratorio Regional de Referencia Virológica (Maracaibo,Venezuela) Rev. Gastroenterol. Perú 2005; 25: 248-253
Pujol F, Rodríguez I, Martínez N, Borberg C, et al. Viral Hepatitis serological markers among pregnant woman in Caracas, Venezuela: implication for perinatal transmition of hepatitis B and C. GEN.1994 ;48:(1)25-28
Nunzio J, Brito J, Brazon S, Carpio C, Ledezma E, Pujol F. Prevalencia de marcadores serológicos para hepatitis B y C en mujeres gestantes del estado Anzoátegui. GEN.1997;51:(3)226-29
Lizarzabal M, Márquez A, Gómez M. Marcadores serológicos de Hepatitis B y C en embarazadas del Estado Zulia. GEN 2010; 64(1):19-20
Garassini M, Pulgar Y, Alvarado M, Garassini M. Hepatitis por Virus C. Factores de riesgo. GEN. 1995;49:(3)189-95
Montes H, Berruela L, Cova J, et al. Prevalencia de anticuerpos contra el virus de la hepatitis C en pacientes multitransfundidos. GEN.1995;49:(2)132-39
Alvarez J, Vancampenhoud M, Comegna M, et al. Hepatitis C: seroprevalencia en niños poli transfundidos. GEN. 1996; 50:(3)142-46
Romero M, Lara D, Garassini M, Falcone M, Lara J, Lecuna V. Marcadores virales para virus B y C en pacientes con hemoglobinopatías. GEN. 1999;53:(2) 69-2
Muller G, Zabaleta M, Colmenarez C, Carriles F, Bianco N, Machado I. Risk Factors for diálisis associated Hepatitis C in Venezuela. Kidney Internacional 1992; 4: 1055-58
Cova J, Rangel A, Montes H, Hernández M. Anticuerpos anti HVC en insuficientes renales crónicos. GEN.1996;50:(1)16-21
Garassini M, Ortega F, Valdés A, et al. Anticuerpos contra el virus de hepatitis C en pacientes con hepatopatías y en sujetos a riesgo. Comunicación preliminar. GEN. 1990;44:(4):343-48
Pujol F, Ponce M, Lema M et al. High incidence of hepatitis C virus infection in hemodiálisis patients in units with high prevalence. Journal of Clinical Microbiology.1996;34:(7) 1633-36.
Machado I, Febres C, Carrasquel E, Llanos L, Martínez C, Vivas B, Vargas F. Hemodiálisis-associated hepatitis C in Venezuela: 10 years follow up. Liver International 2004;24: (supl 4): 56
Peñaloza O, Angulo E, Labrador C, Márquez R, Vivas J, Castro D. Prevalencia de inmunomarcadores para infección por hepatitis viral y VIH en sujetos con insuficiencia renal crónica terminal dependientes de diálisis en un centro de referencia de enfermedades gastrointestinales. GEN. 2000;54: (3):217
Garassini M, Ortega F, Valdés A, et al. Anticuerpos contra el virus de hepatitis C en pacientes con hepatopatías y en sujetos a riesgo. Comunicación preliminar. GEN. 1990;44:(4):343-48
Betancourt C, Carneiro M, Velásquez O, y col. Seroprevalencia del anti HVC en Ciudad Guayana, Estado Bolívar. Comunicación preliminar. GEN. 1992; 46:(3):268
León R, Gamboa A, Quiroz E, Hinestroza H, Lecuna V et al. seroprevalencia de anticuerpos anti-virus de hepatitis C en trabajadores de la salud y empleados de instituciones sanitarias en Venezuela. Informe Preliminar. GEN. 2003;57: (especial): E12-E17.)
Betancourt C. Encuesta personal, Ciudad Guayana 2005. Información personal no publicada
Peña Pacheco, Lisett. Trabajo de grado para optar al título de Licenciada en Bioanálisis de Br. Joanna Lisett Peña Pacheco, Universidad de Oriente, Ciudad Bolívar, Marzo 2006
Marrero C, Albornoz A, Rodríguez L, Pacheco M, Rodríguez C. El tatuaje corporal como factor de riesgo para la transmisión de la infección por el virus de la hepatitis C. GEN. 1997; 51:(4):277-280
Matos C, González K, Lobaton D, Osorio P, Moncada P. Frecuencia de anticuerpo Anti-HCV y tatuaje corporal en miembros del personal de la Armada, Comando Fluvial, Ciudad Bolívar. Noviembre 2003-Junio 2004. XXVII Congreso Venezolano de Gastroenterología, Caracas 2006
Vetencourt R, De Armas J, Vetencourt M. Prevalencia del anticuerpo del virus C en pacientes con enfermedades crónicas hepáticas AgsHB negativos: niños, prostitutas y homosexuales. GEN. 1990;44:(4) 340-52
Mora O, Rodríguez De León L, Bongioanni H, Bolívar F, Wever W, Delgado F. Incidencia del Virus de la Hepatitis C en una población de alto riesgo. GEN 2008; 62(4): 296-299
Camejo M, Mata G, Diaz M. Prevalence of hepatitis B, hepatitis C and syphilis in female sex workers in Venezuela. Rev Saude Publica. 2003;37(3):339-44
De Olival C, Castillo S, Saporitti R, Zabaleta P, Villalobos I. Caracterización epidemiológica de los pacientes infectados por Virus de la Hepatitis C en el Estado Aragua 2002-2007. GEN 2009; 63 (4):258-261
Monsalve-Castillo F, Chacín-Bonilla L, Atencio R, Porto L, Costa-León L, Estévez J, Callejas-Valero D. Baja prevalencia de la infección por el virus de la hepatitis C en una población de reclusos, Maracaibo, Venezuela. Biomédica 2009;29:647-52
Luna M, De Guglielmo, Garassini M, Perrone M, Correnti M. Acta Odontol. Venez 2008;46(3):269-272
Monsalve F, Chacín L, Atencio R, Porto L, Costa L, Echevarría J. Low prevalence of hepatitis C virus infection in Amerindians from Western Venezuela. Mem Inst Oswaldo Cruz 2007; 102(1): 107-110
Fortes M, Trómpiz A, Canónico Y, Vargas B, Machado I. La frecuencia del genotipo 1 del virus de hepatitis C no ha variado en Venezuela. Rev Col Gastroenterol 2009; 24 (3):256-258
Pujol FH, Loureiro CL, Devesa M, Blitz L, Parra K, Beker S, et al. Determination of genotypes of Hepatitis C Virus in Venezuela by restriction fragment length polymorphism. Journal of Clinical Microbiology. 1997; 35:(7) 1870-72
Machado I, León R, Golindano C, Dagher L, Vetencourt R, Garassini M, Poleo J, Tassinari P y col. Genotipos y cuantificación de ARN-VHC en el abordaje clínico y terapéutico de la hepatitis crónica por virus C en Venezuela. GEN 2003; 57: (especial): E40-E44
Venezuela, Ministerio del Poder Popular para la Salud. Anuario Estadístico 2009, Caracas, pág. 41 http://www.bvs.gob.ve/anuario/Anuario2009.pdf. accesado 26 Jun 2016
Venezuela, Ministerio del Poder Popular para la Salud. Anuario de Mortalidad 2010, Caracas 2012: pág. 18
http://www.ovsalud.org/descargas/publicaciones/documentos-oficiales/Anuario-Mortalidad-2010.pdf. accesado 26 Jun 2016
Venezuela, Ministerio del Poder Popular para la Salud, Anuario de Morbilidad 2011: pág. 42. Caracas
http://www.ovsalud.org/descargas/publicaciones/documentos-oficiales/Anuario-Morbilidad-2011.pdf. accesado 26 Jun 2016
Venezuela, Ministerio del Poder Popular para la Salud, Anuario de Morbilidad 2012: pág. 396, Caracas
http://www.ovsalud.org/descargas/publicaciones/documentos-oficiales/Anuario-Morbilidad-2012.pdf. accesado 26 Jun 2016
Venezuela. Ministerio del Poder Popular para la Salud. Boletín Epidemiológico. Semana Epidemiológica Nº 08, 17 al 23 Febrero del 2013. Año LXIII. Págs. 3 y 20 http://www.bvs.gob.ve/boletin_epidemiologico/Boletin_08_2013.pdf. accesado 26 Jun 2016
Venezuela. Ministerio del Poder Popular para la Salud, Boletín Epidemiológico, Semana Epidemiológica Nº 06, 02 al 08 Febrero del 2014. AñoLXIV.Págs. 5 y 22 http://www.bvs.gob.ve/boletin_epidemiologico/Boletin_06_2014.pdf. accesado 26 Jun 2016
Venezuela. Ministerio del Poder Popular para la Salud. Boletín Epidemiológico. Semana Epidemiológica Nº 26, 28 de Junio al 04 de Julio del 2015. Año LXV.Págs. 4 y 27 http://www.bvs.gob.ve/boletin_epidemiologico/Boletin_26_2015.pdf. accesado 26 Jun 2016
Kershenobich D, Razavi H, Sanchez-Avila JF, Bessone F, Coelho HS, Dagher L., et al. Trends and projections of HCV epidemiology in Latin America. Liver International, 2011; 31 (S2): 18-29
Kershenobich D, Razavi H, Curtis CL, Alberti A., et al. Applying a system approach to forecast the total hepatitis C virus-infected population size: model validation using US data. Liver International, 2011; 31 (S2): 4-17
Venezuela, Instituto Nacional de Estadística, http://www.ine.gov.ve, accesado 26 Jun 2016
Fernández S, Pernalete B, Sénior M, Escalante N. ¿Qué sabemos de Hepatitis en Venezuela?. GEN 2010; 64(3):170-173
Escalante N. Hepatitis C: ¿Es adecuado el nivel de conocimiento entre médicos residentes e internos? GEN 2008; 62(3):186-190
Manuel Carreiro. Conferencia magistral Simon Beker 2010. Dr. Cristobal Betancourt ¿Que hemos enseñado en Hepatologia? (4) (Video file) 24 de Diciembre de 2010 [Consultado el 22 de Febrero de 2016] [10:00] Disponible en: https://youtube.com/watch?v=09feaarbR30&sns=em
Sociedad Venezolana de Gastroenterología, Estatutos y Reglamentos, Caracas 2009, Objetivos: págs. 1 y 2. http://www.sovegastro.org/pdf/estatutos_reglamentos(2009).pdf accesado 22 de Febrero de 2016
Vetencourt M. Epidemiologia del VHC en Venezuela. GEN 2005;1 (Suplemento Especial): 4-6
Ghany M.G., Strader D.B., Thomas D.L., Seeff L.B. American Association for the Study of Liver Disease (AASLD). Diagnosis, Management and Treatment of Hepatitis C: an update. Hepatology 2009; 49 (4): 1335-74. http://dx.doi.org/10.1002/hep.22759.
AASLD/IDSA Guidance Panel. Hepatitis C Guidance: AASLD-IDSA Recomendations for Testing, Managing, and Treating Adults Infected with Hepatitis C Virus. Hepatology. 2015; 62: 932-54. http://wwwhcvguidelinesorg
EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015; 63 (1): 199–236.
Fortes M., Trómpiz A., Canónico Y., Vargas B., Machado I. La frecuencia del genotipo 1 del virus de hepatitis C no ha variado en Venezuela. Rev Col Gastroenterol. 2009; 24 (3):256-258.
Machado I., León R., Golindano C., Dagher L., Vetencourt R., Garassini M., y col. Genotipos y cuantificación de ARN-VHC en el abordaje clínico y terapéutico de la hepatitis crónica por virus C en Venezuela. GEN. 2003; 57: (especial): E40-E44.
Méndez-Sánchez N., Paraná R., Cheinquer H., Alves de Mattos A., Gadano A., Silva M., et al. Latin American Association for the Study of the Liver. Recommendations on Treatment of Hepatitis C. Methods for staging liver disease in chronic hepatitis C. Ann Hepatol, 2014; 13 (Suppl. 2): s14-s15.
Dhingra S., Ward S.C., Thung S.N. Liver pathology of hepatitis C, beyond grading and staging of the disease. World J Gastroenterol. 2016; January 28; 22(4): 1357-1366
EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatology. 2015; 63: 237-264.
Cequera A., García de León Méndez M.C. Biomarkers for liver fibrosis: Advances, advantages and disadvantages. Rev Gastroenterol Méx. 2014; 79 (3):187-199.
AASLD-IDSA: Recommendations for Testing, Managing and Treating Hepatitis C, April 2016 version. http://www.hcvguidelines.org.
Séne D, Ghillani-Dalbin P, Thibault V, Guis L, Musset L, et al. Long-term course of mixed cryoglobulinemia in patients infected with hepatitis C virus. The Journal of Rheumatol. 2004; 31:2199-206.
Palazzi C, D’Amico E, D’Angelo S, Gilio M and Olivieri I. Rheumatic manifestations of hepatitis C virus chronic infection: Indications for a correct diagnosis. World J Gastroenterol. 2016; 22(4): 1405-10.
Cacoub P., Renou C., Rosenthal E., Cohen P., Loury I., et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective munlticenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medicine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine. 2000, Jan; 79:47-56.
Cacoub P, Comarmond C, Domont F, Savey L, Desbois A et al. Extrahepatic manifestations of chronic Hepatitis C virus infection. Ther Adv Infect Dis; 2016; 3(1): 3-14.
Narciso-Schiavon J. and Lucca Schiavon L. Autoantibodies in chronic hepatitis C: A clinical perspective. World J Hepatol. 2015; 7(8): 1074-85.
Trómpiz Araque A.C., Fortes Soto M.P., Vargas-Lovelle B., Machado Bártoli I.V. Aplicación de un algoritmo sero-virológico en el diagnóstico de la infección crónica por el virus de la hepatitis C en Venezuela. Rev Panam Infectol. 2010; 12(4):18-21.
Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010, Lozano R, Naghavi M et al.Lancet 2012;380:2095-2128
Zhang M, Rosenberg PS, Brown DL, et al. Correlates of spontaneous clearance of hepatitis C virus among people with hemophilia. Blood 2006; 107: 892-897
Beinhardt S, Payer BA, Datz C, et al. A diagnostic score for the prediction of spontaneous resolution of acute hepatitis C virus infection. J Hepatol 2013; 59: 972-977
Mangia A, Santoro R, Copetti M, Massari M, Piazzolla V, Spada E, et al. Treatment
optimization and prediction of HCV clearance in patients with acute HCV infection. J
Hepatol 2013;59:221–228.
Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and Spontaneous clearance of hepatitis C virus. Nature 2009; 461: 798-801.
Deterding K, Gruner N, Buggisch P, Wiegand J, Galle PR, Spengler U, et al.Delayed versus immediate treatment for patients with acute hepatitis C: arandomised controlled non-inferiority trial. Lancet Infect Dis 2013;13:497–506 1.
Lawitz E, Gane EJ. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;369(7):678-679.
Mishra P, Florian J, Qi K, et al. FDA perspective on sofosbuvir therapy for patients with chronic hepatitis C virus genotype 1 infection who did not respond to treatment with pegylated interferon and ribavirin. Gastroenterology. 2014;147(6):1196-1200.
Younossi ZM, Bacon BR, Dieterich DT, et al. Disparate access to treatment regimens in chronic hepatitis C patients: data from the TRIO network. J Viral Hepat. 2016;23(6):447-454.
Jacobson IM, Dore GJ, Foster GR, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384(9941):403-413.
Manns M, Marcellin P, Poordad F, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384(9941):414-426.
Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology. 2014;146(7):1669-1679.e1663.
Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-Elbasvir Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial. Ann Intern Med. 2015;163(1):1-13.
Sulkowski M, Hezode C, Gerstoft J, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385(9973):1087-1097.
Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898.
Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594-1603.
Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756-1765.
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211-221.
Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867-1877.
Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993-2001.
Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373(27):2608-2617.
Younossi ZM, Stepanova M, Feld J, et al. Sofosbuvir/velpatasvir improves patient-reported outcomes in HCV patients: Results from ASTRAL-1 placebo-controlled trial. J Hepatol. 2016;65(1):33-39.
Feld JJ, Jacobson IM, Hezode C, et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015;373(27):2599-2607.
Foster GR, Pianko S, Brown A, et al. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology. 2015;149(6):1462-1470.
Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61(4):1127-1135.
Moreno C, Hezode C, Marcellin P, et al. Efficacy and safety of simeprevir with PegIFN/ribavirin in naive or experienced patients infected with chronic HCV genotype 4. J Hepatol. 2015;62(5):1047-1055.
Hezode C, Asselah T, Reddy KR, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet. 2015;385(9986):2502-2509.
Kohli A, Kapoor R, Sims Z, et al. Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis. 2015;15(9):1049-1054.
Gane EJ, Hyland RH, An D, et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology. 2015;149(6):1454-1461.e1451.
Westbrook RH1, Dusheiko G2. Natural history of hepatitis C.J Hepatol. 2014 Nov;61(1 Suppl):S58-68.
Morgan RL, Baack b, Snith BD, Yartel A, Pitasi M, Falck Itter. Erradication of hepatitis C virus infection and the development of hepatocelular carcinoma: a metaanalysis of obsertional studies. Ann Internal Med 2013; 158: 329-33.
Bruno S, Crosignani A, Facciotto C, Rossi S, Roffi L, Redaelli A, de Franchis R, Almasio PL, Maisonneuve P. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology 2010;51(6):2069-76.
Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20): 1889-98.
Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014; 370(16): 1483-93.
Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrosis. N Engl J Med. 2014; 370(21):1973-82
Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, et al. Simeprevir plus Sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferón and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 2014;384:1756-1765.
Lawitz E, Matusow G, DeJesus E, Yoshida EM, Felizarta F, Ghalib R, Godofsky E,et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrosis: a Phase 3 study (OPTIMIST-2). Hepatology. 2015. Doi:10.100/hep.28422. Epub ahead of print)
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014 Jan 16;370(3): 211-21.
Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, et al. Daclatasvir, Sofosbuvir, and Ribavirin combination for HCV patients with advanced cirrosis or posttransplant recurrence: Phase 3 ALLY-1 Study. Hepatology. 2016; 63 (5): 1493-505.
Lawitz E, Gane E, Pearlman B, Tam E, Ghesquiere W, Guyader D, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir and elbasvir with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrosis and patients with previous null response with or without cirrosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385 (9973): 1075-86
Feld JJ, Moreno C, Trinh R, Tam E, Bourgeois S, Horsmans Y, et al. Sustained virologic response of 100% in HCV genotype 1b patients with cirrhosis receiving ombitasvir/paritaprevir/r and dasabuvir for 12weeks. J Hepatol. 2016 Feb;64(2):301-7.
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2011; 55: 245-264.
American Association for the Study of Liver Disease AASLD Hepatitis C Guidance. Hepatology 2015; 62: 932-956.
Lawitz, E., Mangia, A., Wyles, D., Rodriguez-Torres, M, Hassanein, T., Gordon, S., et al. Sofosbuvir for Previously Untreated Chronic Hepatitis C Infection. N Engl J Med 2013; 368:1878-87.
Jacobson, I., Gordon, S., Kowdley, K., Yoshida, E., Rodriguez-Torres, Sulkowski, M., et al. Sofosbuvir for Hepatitis C Genotype 2 or 3 in Patients without Treatment Options. N Engl J Med 2013; 368:1867-77.
Foster GR, Irving WL, Cheung MC, et al. Cohort study of the impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 2016; 64(6): 1224-1231.
Lawitz E, Poordad F, Brainard DM, Hyland RH, An D, Symonds WT, et al. Sofosbuvir in combination with PegINF and Ribavrin for 12 weeks provides high SVR rates in HCV infected genotype 2 o 3 treatment experienced patients with and without compensated cirrhosis: results from the LONESTAR 2 study. Hepatology 2013;58:1380A.
Gambato M, Lens S, Navasa M, Forns X. Treatment options in patients with descompensated cirrhosis, pre – and post –transplantation. J Hepatol 2014; 61: S120-S131.
Afdhal N, Everson G, Calleja JL, McCaughan G, Symonds WT, Denning J, et al. Sofosbuvir and ribavirin for treatment of chronic HCV with cirrhosis and portal hypertension with or without decompensation: early virologic response and safety. J Hepatol 2014; 60: S28.
Mangia A, Arleo A, Copetti M, Miscio M, Piazzolla V, Santoro R, Squillante MM. The combination of daclatasvir and sofosbuvir for curing genotype 2 patients who cannot tolerate ribavirin. Liver Int. 2016 Jan [Epub ahead of print].
Curry M, O’Leary J, Bzowej N, Muir A, Korenblat K. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med 2015; 373:2618-2628.
Nkontchou G1, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, Roulot D, Ganne-Carrie N, Grando-Lemaire V, Trinchet JC, Gordien E, Vicaut E, Baghad I, Beaugrand M. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011 Oct;18(10):e516-22.
Bochud PY1, Cai T, Overbeck K, Bochud M, Dufour JF, Müllhaupt B, Borovicka J, Heim M, Moradpour D, Cerny A, Malinverni R, Francioli P, Negro F; Swiss Hepatitis C Cohort Study Group. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009 Oct;51(4):655-66.
Leroy V, Angus P, Bronowicki JP, Dore GJ, Hezode C, Pianko S, Pol S. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: A randomized phase III study (ALLY-3+). Hepatology. 2016 May; 63(5):1430-41.
Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015b;61(4):1127-1135.
Pol S1, Vallet-Pichard A, Corouge M.Treatment of hepatitis C virus genotype 3-infection. Liver Int. 2014 Feb;34 Suppl 1:18-23.
Hezode C, Ledinghen V, Fontaine H. et al. Daclatasvir plus sofosbuvir with or without ribavirin in genotype 3 patients from a large French multicenter compassionate use program. 66th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). November 13-17, 2015b; San Francisco, CA
Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. The New England Journal of Medicine 2014b;370:1993-2001.
Poordad F, Lawitz E, Gutierrez JA, et al. C-SWIFT: grazoprevir/elbasvir + sofosbuvir in cirrhotic and noncirrhotic, treatment-naive patients with hepatitis C virus genotype 1 infection for durations of 4, 6 or 8 weeks and genotype 3 infection for durations of 8 or 12 weeks J Hepatol. 2015;62(1)(suppl).
Nguyen MH, Keeffe EB. Prevalencia y tratamiento de los genotipos 4, 5 y 6 del Virus de la hepatitis C. Clínica de Gastroenterología y Hepatología. 2005:3:S97-S101.
Aidsinfonet.org (2014). Los Genotipos de Hepatitis C. Disponible: http://www.aidsinfonet.org/
Corouge M, Vallet-Pichard A, Pol. HCV and the kidney. Liver Int 2016 Jan;36 Suppl 1:28-3
Mesquita F, Santos ME, Benzaken A, Correa RG, Catapan E, Sreno LS, Naviera MC. The Brazilian comprehensive response to hepatitis C: form strategic thinking to access to interferon-free therapy. BMC Public Health. 2016; 16(1): 1132
Ferenci, P. Treatment of hepatitis C in difficult-to-treat patients. Nat. Rev. Gastroenterol. Hepatol. 12, 284–292 (2015)
Carvalho-Filho RJ, Feldner AC, Silva AE et al. World J Gastroenterol 2015 14; 21(2): 408-422
Torres H, Mahale P, Blechacz B, Miller E, Kaseb A, H. Herlong F, et al. Effect of Hepatitis C Virus Infection in Patients With Cancer: Addressing a Neglected Population. J Natl Compr Canc Netw 2015;13:41-50
Peffault de Latour R, Lévy V, Asselah T, Marcellin P, Scieux C, Adès L, Traineau R, et al. Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood 2004 103:1618-1624;
Klingemann HG, Grigg AP, Wilkie-Boyd K, Barnett MJ, Eaves AC, Reece DE, Shepherd JD and Phillips GL. Treatment with recombinant interferon (alpha-2b) early after bone marrow transplantation in patients at high risk for relapse. Blood. 1991;78(12):3306-11
Kyvernitakis A, Mahale P, Popat U, Jiang Y, Hosry J, Champlin R, Torres H. Hepatitis C Virus Infection in Patients Undergoing Hematopoietic Cell Transplantation in the Era of Direct-Acting Antiviral Agents. Biol Blood Marrow Transplant, 2016;22(4):717-22
Mahale P, Kontoyiannis P, Chemaly R, Jiang Y, Hwang J, Davila M, Torres H. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol 2012;57:1177–85
Mahale P, Thomas S, Kyvernitakis A and Torres H. Management of Multiple Myeloma Complicated by Hepatitis C Virus Reactivation: The Role of New Antiviral Therapy. Open Forum Infect Dis. 2016) 3 (1): doi: 10.1093/ofid/ofv211
Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000 Mar. 31(3):751-5.
Tovo, P. Calitri,C. Scolfaro, C et al. Vertically acquired hepatitis C virus infection:
Correlates of transmission and disease progression. World Journal of
Gastroenterology, 2016; 22(4):1362- 1392
Sasaki KJ. Liver Disease and Pregnancy. [updated 2015 Aug 27].Available from: URL: http://emedicine.medscape.com/article/188143-overview#a1
Palmer, M. Guide of Hepatitis and Liver Disease. On Line. Publicado 2004 https://www.amazon.com/Melissa-Palmers-Guide-Hepatitis-Disease/dp/1583331883
Praveen K Roy. Liver Disease and Pregnancy. eMedicine Specialties Article Last Updated: Jan 16, 2008.
Giakoumelous. N, Human Reproduction update. 22(1): 115-133. 2106
Yang, L. Zhao, R. Song, X. Int. J. Clinic Exp Med 8(4): 6.230-6.235. 2.015
Huang, QT. Huang Q. Zhong M, et al. J. Viral Hepathology 22(12); 1.033-
042. 2.015
Balistreni, W. Current Emerging Treatment Strategies. The Liver Meeting 2.014.
American Association for the study of Liver Diseases (AASLD)
García- Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M et al.
Hepatitis C virus kinetics during and immediately after liver transplantation.
Hepatology 2002;35 :680-687
Gane E, Pilmore H. Management of chronic viral hepatitis before and after renal
transplantation. Transplantation 2002 ;74 :427-437
Curry MP, Forns X, Chung RT, Terrault NA, Brown Jr R, Fenkel JM, et al.
Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver
transplantation: an open-label study. Gastroenterology 2015 :148: 100- 107
Flamm SL, Everson GT, Charlton M, Denning JM, Arterburn S, Brandt-Sarif T, et
al. Ledipasvir/sofosbuvir with ribavirin for the treatment of HCV in patients with
decompensated cirrhosis: preliminary results of a prospective, multicenter study.
Hepatology 2014; 60: 320A.
Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-
/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis . N Engl
J Med 2014; 370: 1973-1982
Jensen DM, O´Leary JG, Pockros PJ, Sherman KE, Kwo PY, Mailliard ME, et al.
Safety and efficacy of sofosbuvir-containing regimens for hepatitis C: real- word
experience in a diverse, longitudinal observational cohort. Hepatology 2014;
: 219 A
Forman LM, Lewis JD, Berlin JA, Feldman HL, Lucey MR. The association
between hepatitis C infection and suvival after orthotopic liver transplantation.
Gastroenterology 2002; 122: 889-896
Prieto M, Berenguer M, Rayon JM, Cordoba J, Arguello L, Carrasco D, et al.
High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection
following transplantation: relationship with rejection episodes. Hepatology 1999;
:250-256
Blasco A, Forns X, Carrion JA, García-Pagan JD, Gilabert R, Rimola A et al.
Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C
recurrence after liver transplantation. Hepatology 2006; 43: 492-499
Neumann UP, Berg T, Bahra M, Seehofer D, Langrehr JM, Neuhaus R, et al.
Fibrosis progression after liver transplantation in patients with recurrent hepatitis
C. J Hepatol 2004 ;41: 830-836.
Berenguer M, Palau A, Aguilera V, Rayon JM, Juan FS, Prieto M. Clinical
benefits of antiviral therapy in patients with recurrent hepatitis C following liver
transplantation. Am J Transplant 2008; 8: 679-687.
Gambato M, Lens S, Navasa M, Forns X, Treatment options in patients with
decompensated cirrhosis, pre- and post transplantation. J Hepatol 2014; 61:
S120-S131.
Hoofnagle J. Hepatic decompensation during direct-acting antiviral therapy of chronic hepatitis C. J Hepatology 2016; 64: 763–765
Valled A, Pol S. Hepatitis viruses and human immunodeficiency virus coinfection: pathogenesis and treatment. J Hepatology. 2004 (41): 156-166
Wedwmwyer H, Duberg A, Buti M. et al. Strategies to manegement Hepatitis C virus (HCV) disease burden. Journal of Viral Hepatitis. 2014; 21( suppl 1): 60-89.
Benhamoud Y, Bochet M, Di Martino V, Charlotte F, Azria F, Cutellier A, et al. Liver fibrosis progression in human immunodefiency virus and Hepatitis C virus coinfected patients. The Multiviric Group. Hepatology 1999; 30: 1054-1058
Allen J, Smith C, Bhagani S. Will antiretroviral therapy reduce HIV-related liver risk? Curr Opin HIV AIDS. 2014; 9: 48-53
Hernández M, Sherman K. HIV/Hepatitis C coinfection natural history and disease progression. Curr Opin HIV AIDS. 2011; 6: 478-482
Rockstroh K. Optimal therapy of HIV/HCV co-infected patients with direct acting antivirals. Liver International 2015; 35 (suppl 1): 51-55
Kumar S, Jacobson I. Antiviral therapy with nucleotide polymerase inhibitors for chronic hepatitis C. J Hepatology 2014 (61): 591-597.
Mara W, Georg D, Zeuzem S. Interferon-free antiviral combination therapies without nucleosidic polymerase inhibitors. J Hepatology 2014 (61): s98- s107.
Lawitz E., Mangia A, Wyles D, Rodriguez-Torres, M,Hassanein T, Gordon S, et al. Sofosbuvir for previously untreated chronic Hepatitis C infection. N Eng J Med 2013;368:1878-1887
Bunchorntavakul C, Tanwandee T. Treatment of Chronic Hepatitis C in Special Populations. Gastroenterol Clin N Am. 2015; 44: 883–900
Dyson J, et al. Liver toxicity associated with Sofosbuvir, an NS5A inhibitor and Ribavirin use. J Hepatology. Vol 64 pp 234-238. 2016
EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol (2016), http://dx.doi.org/10.1016/j. jhep.2016.09.001
Patel N, Nasiri M, Koroglu A, Amin R, McGuey L, McNutt L-A, et al. Prevalence of drug-drug interactions upon addition of simeprevir- or sofosbuvir-containing treatment to medication profiles of patients with HIV and hepatitis C coinfection. AIDS Res Hum Retroviruses 2015;31(2):189-97.
Luetkemeyer AF, McDonald C, Ramgopal M, Noviello S, Bhore R, Ackerman P. 12 Weeks of Daclatasvir in Combination With Sofosbuvir for HIV-HCV Coinfection (ALLY-2 Study): Efficacy and Safety by HIV Combination Antiretroviral Regimens. Clin Infect Dis Off Publ Infect Dis Soc Am 2016;62(12):1489-96.
Khatri A, Dutta S, Wang H, Podsadecki T, Trinh R, Awni W, et al. Evaluation of Drug-Drug Interactions Between Hepatitis C Antiviral Agents Ombitasvir, Paritaprevir/Ritonavir, and Dasabuvir and HIV-1 Protease Inhibitors. Clin Infect Dis Off Publ Infect Dis Soc Am 2016;62(8):972-9.
Menon RM, Badri PS, Wang T, Polepally AR, Zha J, Khatri A, et al. Drug-drug interaction profile of the all-oral anti-hepatitis C virus regimen of paritaprevir/ritonavir, ombitasvir, and dasabuvir. J Hepatol 2015;63(1):20-9.
Chauvin B, Drouot S, Barrail-Tran A, Taburet A-M. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet 2013;52(10):815-31.
Berecz R, Dorado P, De La Rubia A, Cáceres MC, Degrell I, LLerena A. The role of cytochrome P450 enzymes in the metabolism of risperidone and its clinical relevance for drug interactions. Curr Drug Targets 2004;5(6):573-9.
Renet S, Chaumais M-C, Antonini T, Zhao A, Thomas L, Savoure A, et al. Extreme bradycardia after first doses of sofosbuvir and daclatasvir in patients receiving amiodarone: 2 cases including a rechallenge. Gastroenterology 2015;149(6):1378-1380.e1.
Tischer S, Fontana RJ. Drug-drug interactions and Idiosyncratic Hepatotoxicity in the Liver Transplant setting. J Hepatol 2014;60(4):872-84
Fabrizi F, Dixit V, Martin P, Messa P. Hepatitis C Virus Increases the Risk of Kidney Disease Among HIV-Positive Patients: Systematic Review and Meta-Analysis. Journal of Medical Virology. 2016; 88:487–497
Soriano V, Labarga P, Mendoza C, Fernández-Montero J, Esposito I, Benítez-Gutiérrez L, Peña J, Barreiro P. New hepatitis C therapies for special patient populations. Expert Opinion on Pharmacotherapy; 2016, 17 (2): 217–229
Hundemer Gregory et al. Use of Sofosbuvir based acting antiviral therapy for Hepatitis C viral infection in patients with severe renal insufficiency. Infections Disease. Vol 47: 924-8829. 2015
Stine J, Intagliata N, Shah N, et al. Hepatic decompensation likely attributable to simeprevir in patients with advanced cirrhosis. Dig Dis Sci. 2015;60:1031–1035.
Ferenci P, Kozbial K, Mandorfer M, et al. HCV targeting of patients with cirrhosis. J Hepatol. 2015;63:1015–1022.
Stock P, Terrault N. HIV and liver transplantation: Hepatitis C is the last hurdle. Hepatology. 2015;61:1747–1754.
Soriano V, Sherman K, Rockstroh J, Dieterich D, David B, Sulkowski Mark, and Less M. Challenges and opportunities for hepatitis C drug development in HIVHCV coinfected patients. Aids. 2011;25:2197–2208.
Altice F, Kamarulzaman A, Soriano V, Schechter M and Friedland G. Treatment of medical, psychiatric, and substance use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:367–387.
Taylor LE, Swan T, Matthews GV. Management of HCV/HIV coinfection among people who use drugs in the era of direct-acting antiviral–based therapy. Clin Infect Dis 2013; 57(Suppl 2):S118–24.
Checa C, Stoszek, S, Quarleri J, Losso M,Ivalo S, Peixoto M, et al. Mother to Child Transmission of Hepatitis C Virus (HCV) among HIV/HCV coinfection woman. J of the Pediatric Infections Diseases Society. Vol 2 No 2 pp 126-35. 2013.
Beste L, Bondurant H, Ioannou G. Prevalence and management of chronic hepatitis C virus infection in women. Med Clin N Am. 2015;99:575–586.
Bickerstaff C. The cost-effectiveness of novel direct acting antiviral agent therapies for the treatment of chronic hepatitis C. Expert Rev Pharmacoecon Outcomes Res. 2015;15:787–800.
Patel N, Nasiri M, Koroglu A, Amin R, McGuey L, McNutt L-A, et al. Prevalence of drug-drug interactions upon addition of simeprevir- or sofosbuvir-containing treatment to medication profiles of patients with HIV and hepatitis C coinfection. AIDS Res Hum Retroviruses 2015;31(2):189-97.
Luetkemeyer AF, McDonald C, Ramgopal M, Noviello S, Bhore R, Ackerman P. 12 Weeks of Daclatasvir in Combination With Sofosbuvir for HIV-HCV Coinfection (ALLY-2 Study): Efficacy and Safety by HIV Combination Antiretroviral Regimens. Clin Infect Dis Off Publ Infect Dis Soc Am 2016;62(12):1489-96.
Khatri A, Dutta S, Wang H, Podsadecki T, Trinh R, Awni W, et al. Evaluation of Drug-Drug Interactions Between Hepatitis C Antiviral Agents Ombitasvir, Paritaprevir/Ritonavir, and Dasabuvir and HIV-1 Protease Inhibitors. Clin Infect Dis Off Publ Infect Dis Soc Am 2016;62(8):972-9.
Bifano M, Hwang C, Oosterhuis B, Hartstra J, Grasela D, Tiessen R, et al. Assessment of pharmacokinetic interactions of the HCV NS5A replication complex inhibitor daclatasvir with antiretroviral agents: ritonavir-boosted atazanavir, efavirenz and tenofovir. Antivir Ther 2013;18(7):931-40.
Ouwerkerk-Mahadevan S, Snoeys J, Peeters M, Beumont-Mauviel M, Simion A. Drug-Drug Interactions with the NS3/4A Protease Inhibitor Simeprevir. Clin Pharmacokinet 2016;55(2):197-208.
Menon RM, Badri PS, Wang T, Polepally AR, Zha J, Khatri A, et al. Drug-drug interaction profile of the all-oral anti-hepatitis C virus regimen of paritaprevir/ritonavir, ombitasvir, and dasabuvir. J Hepatol 2015;63(1):20-9.
Chauvin B, Drouot S, Barrail-Tran A, Taburet A-M. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet 2013;52(10):815-31.
Berecz R, Dorado P, De La Rubia A, Cáceres MC, Degrell I, LLerena A. The role of cytochrome P450 enzymes in the metabolism of risperidone and its clinical relevance for drug interactions. Curr Drug Targets 2004;5(6):573-9.
Renet S, Chaumais M-C, Antonini T, Zhao A, Thomas L, Savoure A, et al. Extreme bradycardia after first doses of sofosbuvir and daclatasvir in patients receiving amiodarone: 2 cases including a rechallenge. Gastroenterology 2015;149(6):1378-1380.e1.
Tischer S, Fontana RJ. Drug-drug interactions and Idiosyncratic Hepatotoxicity in the Liver Transplant setting. J Hepatol 2014;60(4):872-84
DOI: http://dx.doi.org/10.61155/gen.v71i2.356
IMÁGENES GEN
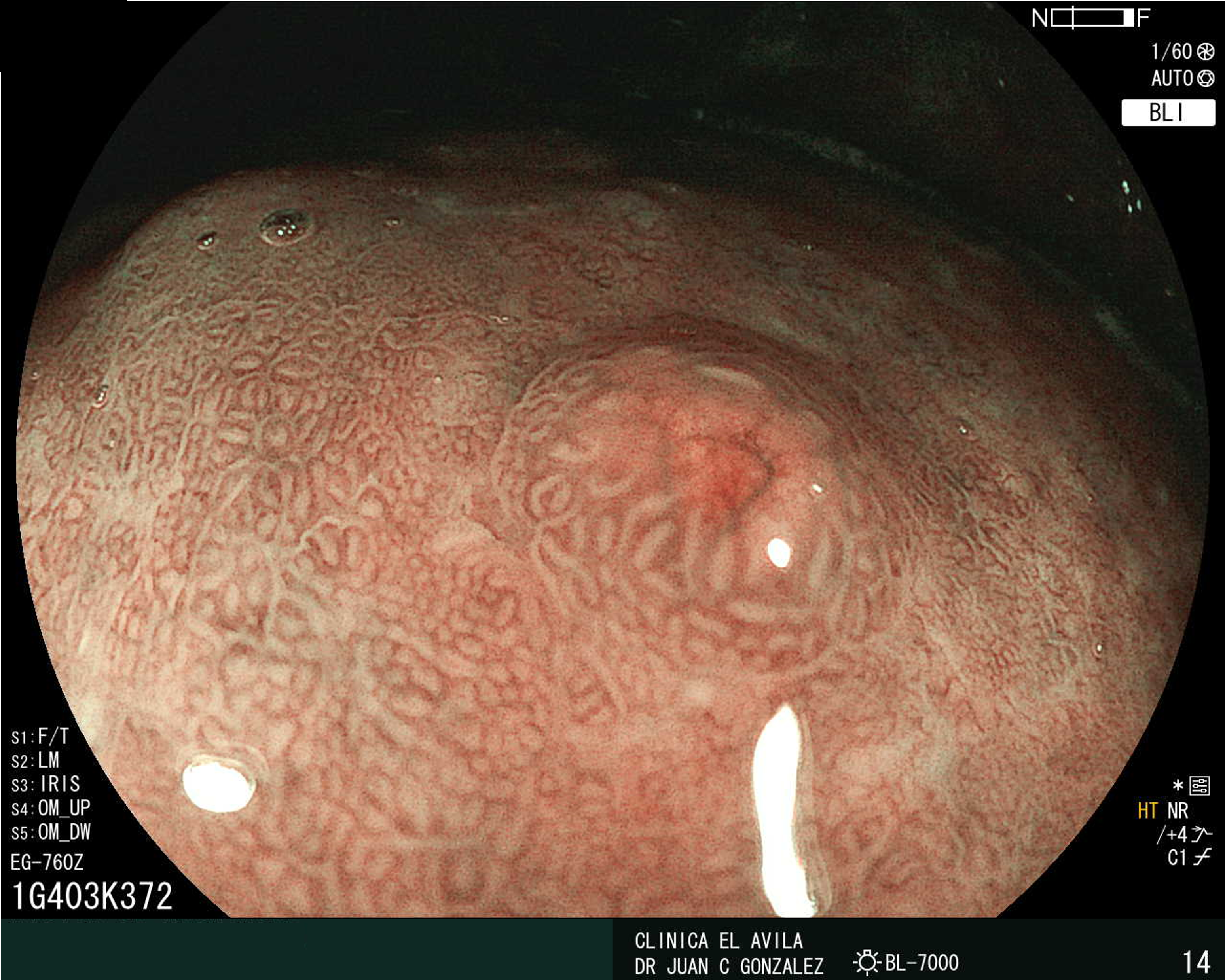
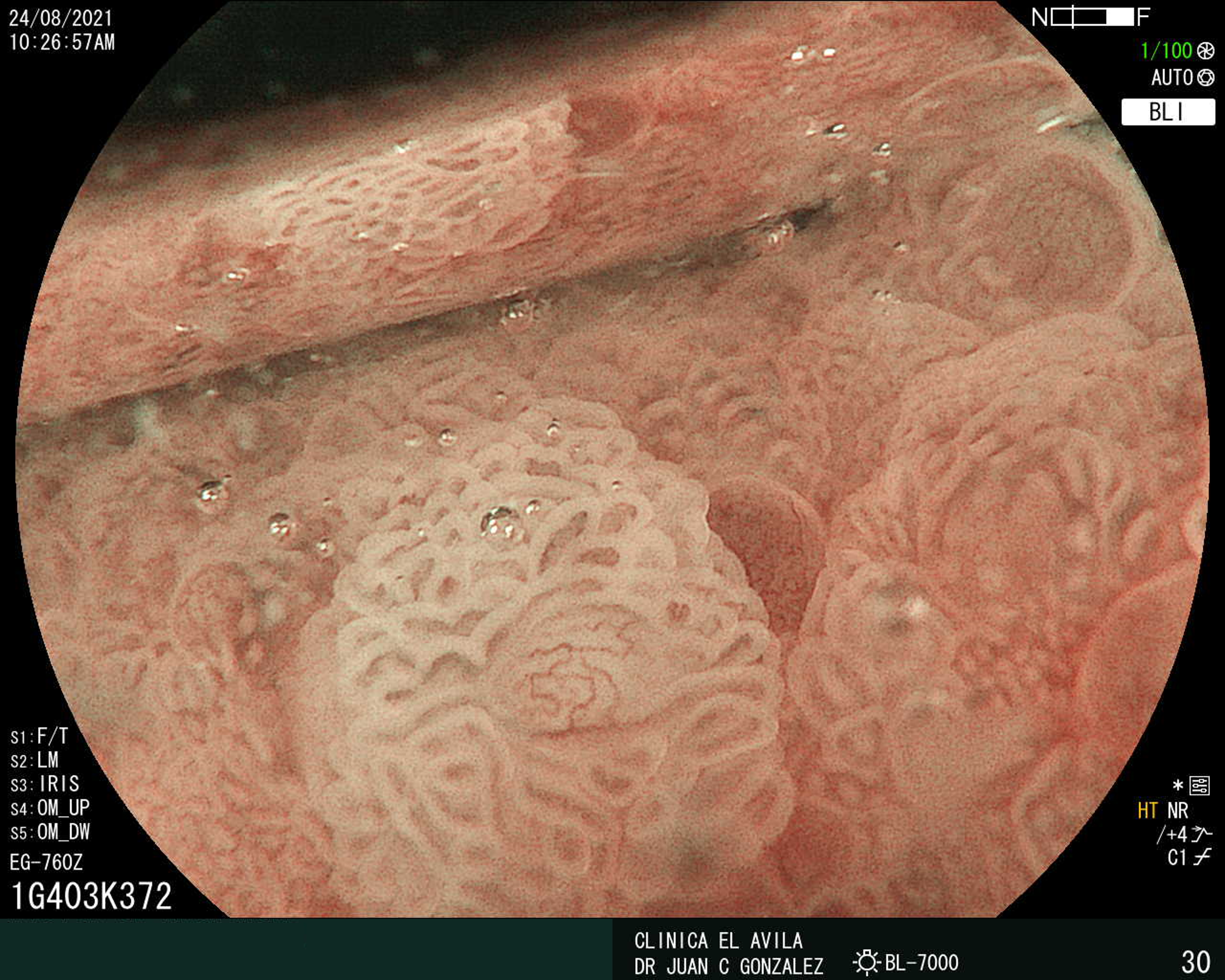
| Figura 1. Tumor Neuroendocrino Gástrico | Figura 2. Hiperplasia de Células Neuroendocrinas en estómago |
 |  |
 |  |  |
ISSN: 0016-3503 e-ISSN: 2477-975X









 ESCUCHAR RESUMEN DEL ARTICULO
ESCUCHAR RESUMEN DEL ARTICULO